When Soda Drinks First Appeared, and How They’re Produced
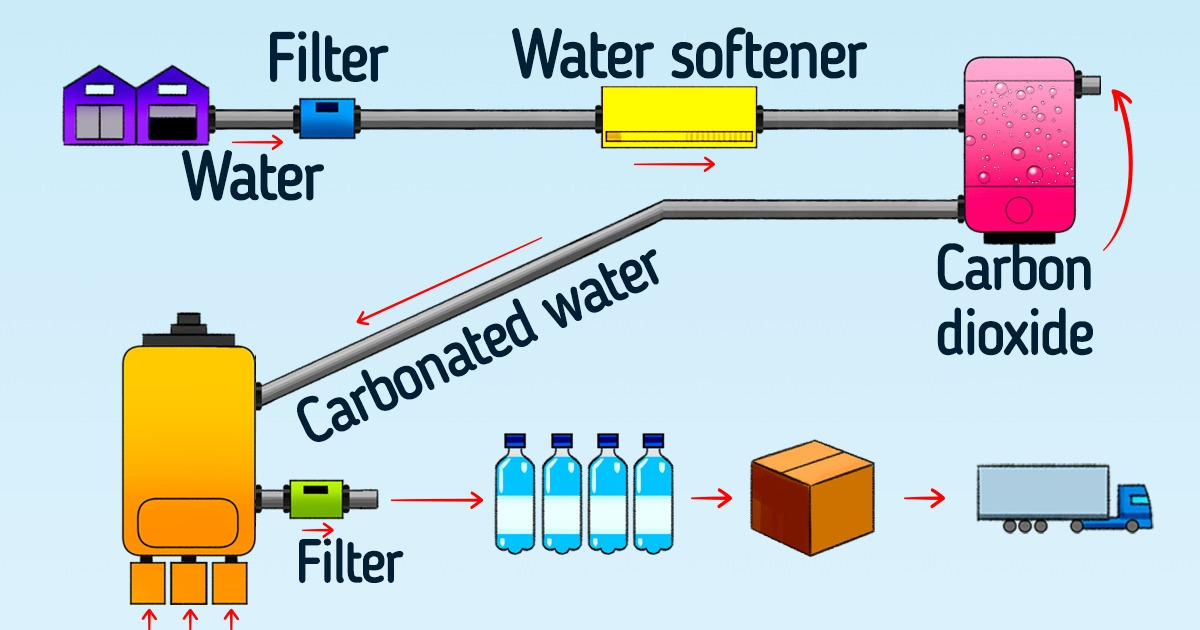
Soda drinks are simply made of ordinary or mineral water saturated by carbon dioxide. It is dissolved in a drink or artificially added to the water under pressure, which causes small bubbles to form in the liquid, and that’s what gives the water that characteristic fizz.
5-Minute Crafts is explaining when soda drinks first appeared, how they are made, and if it’s possible to make your own at home.
When soda drinks first appeared
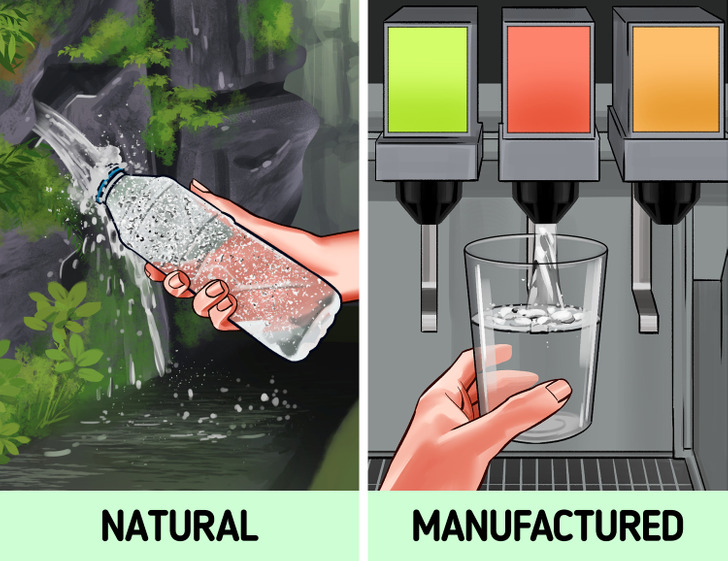
Some natural springs have naturally carbonated water formed in them. Most often, this is due to volcanic activity or special geological conditions of the area. Because of this, water is naturally saturated with carbon dioxide.
Back in the day, such water was extremely popular because of its supposed medicinal properties. The first attempts to produce carbonated drinks were made because of people’s desire to reproduce the naturally fizzy water that was mined at famous European resorts. Early experimenters believed that the bubbles were the source of the healing properties of water, so they concentrated on saturating their product with carbon dioxide.
By the end of the eighteenth century, several researchers had discovered methods for carbonizing water, but the most famous were the experiments done by Joseph Priestley. Invented by him in 1767, the method of saturating water with carbon dioxide soon started to be used in commercial production.
The first factory for producing carbonated drinks was built by the British pharmacist, Thomas Henry. However, the real birth of the new industry happened a bit later when the German scientist, Johann Jacob Schweppe, developed a process to manufacture bottled carbonated mineral water based on the discoveries of Joseph Priestley. In 1783, he founded the Schweppes company that still remains one of the most popular brands of carbonated drinks.
How carbonated drinks are made
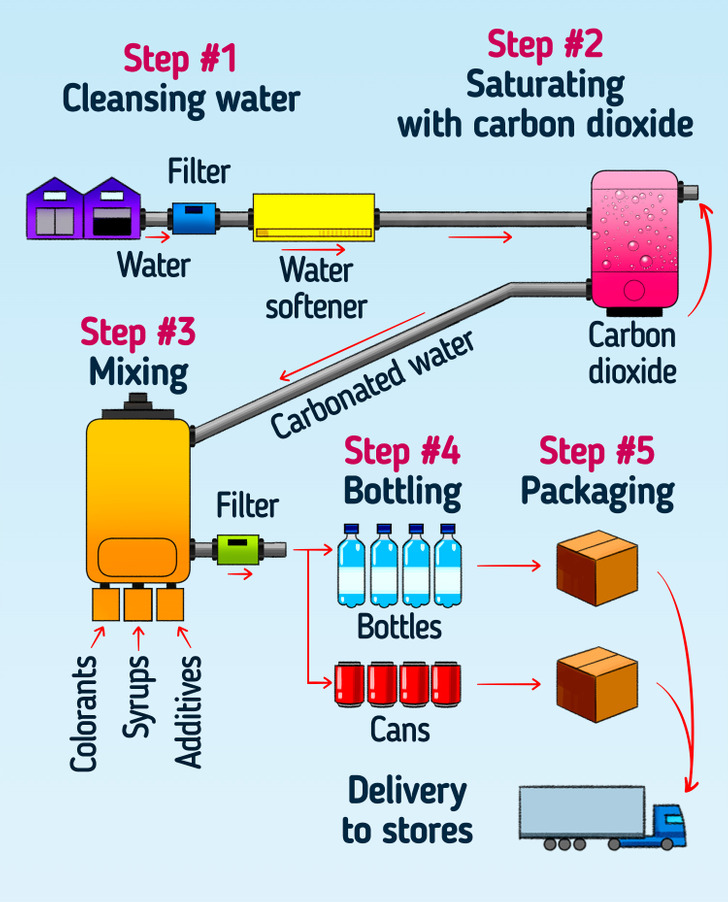
Today, carbonated drinks are made by injecting pressurized carbon dioxide into them. It increases the solubility and saturates the water with more carbon dioxide. When the bottle is opened, the pressure is released, the gas comes out of the drink, creates bubbles, and makes the fizz that we all are so used to.
Carbon dioxide is supplied to the factory in liquid or solid form and then stored in heavy steel containers under high pressure. The solid form, also called dry ice, is simply compressed and frozen carbon dioxide. This form allows making the product more economical to transport.
Many carbonated drinks contain added or dissolved minerals, such as potassium bicarbonate, sodium bicarbonate, or potassium sulfate. They occur naturally in some mineral waters and are added to artificial ones in order to mimic the natural taste and compensate for the acidity of carbon dioxide.
In commercial establishments that use carbonated drinks for producing other soda drinks, the cost of purchasing it pre-made can be very high. Instead, it is produced right on the spot with the help of small mechanical carbonators that also inject pressurized carbon dioxide into the water.
If carbonated drinks can be produced at home

For making home carbonated drinks you can buy special devices.
- A manual siphon looks like a big bottle and has a cartridge with carbon dioxide installed on its side. First, you pour water into the bottle and then optionally add syrups, aromatizers, or fruit flavorings into it. Then you press the lever and pressurized water is poured into a glass, getting saturated with carbon dioxide at the same time. Cartridges for such siphons will have to be changed periodically, and the old ones should be handed over to recycling for special points. In addition, you must strictly follow the instructions for using the siphon, otherwise, the bottle may explode.
- Soda-making machines work by the same principle. A carbon dioxide cylinder is installed in this machine, and a bottle of water is placed into a special compartment. With a simple push of a button, carbon dioxide gets inside the bottle. The only thing you need to do is disconnect the bottle from the machine and enjoy the drink.
What carbonated drinks do you like most?