What Diffusion Is and How It Works
We often associate diffusion with something we learned in school, where bright food coloring is poured into a flask of water, and the liquids mix together nicely. But in fact, there are many more examples of diffusion. For example, cells in living organisms are saturated with oxygen, gas exchange takes place, and nerve impulses propagate with its help.
5-Minute Crafts would like to tell you about what diffusion is, how it works, and how important it is in our life.
What diffusion is
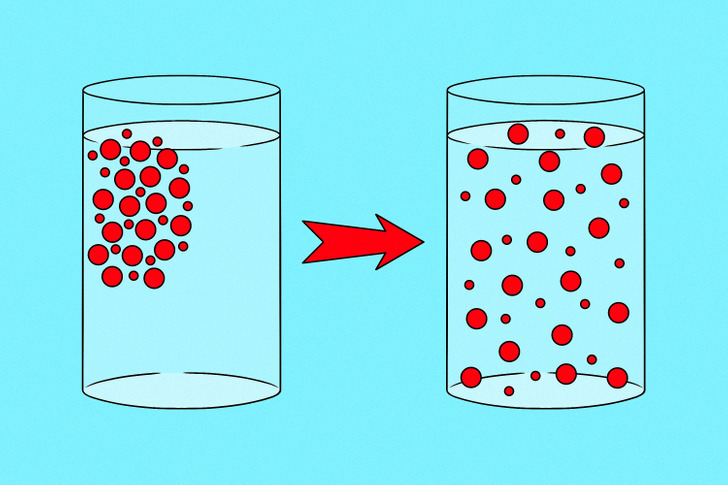
Diffusion is the movement of a substance from regions of higher to regions of lower concentration. Most often, it occurs in liquids and gases, where their particles accidentally collide with each other and move away in different directions.
Imagine someone sprays deodorant on the other side of a room. After some time, the smell reaches you. This happens because the deodorant particles collide with the air particles and scatter throughout the room. As a result, you sense the smell of the deodorant, the particles of which have reached your nose. This process is called diffusion.
How diffusion works
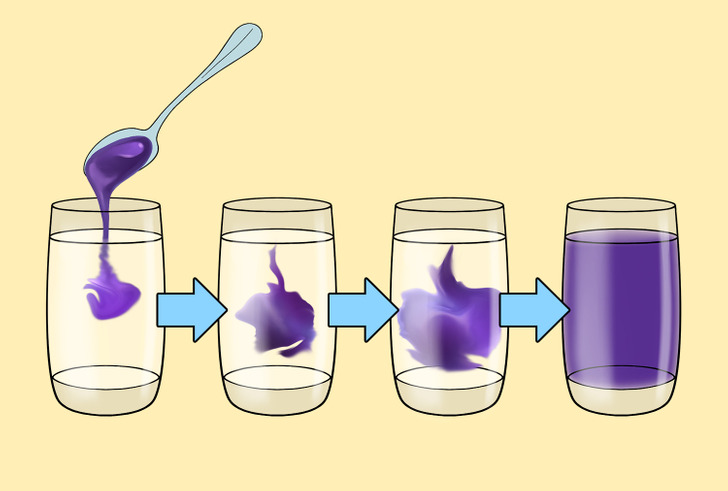
Diffusion can occur in solids, gases, and liquids. The speed of the process depends on the type of substance. So, one gas diffuses into another very quickly. In liquids, on the contrary, it will propagate more slowly, and in solids, the diffusion rate slows down significantly.
To understand this process, imagine mixing water with blueberry syrup. The syrup is quite thick, and has higher concentration than water. In the process of diffusion, the syrup spreads over the space filled with water and partially mixes with it. As a result, it becomes thinner and less concentrated, and the water takes on a distinct blueberry flavor.
What affects diffusion
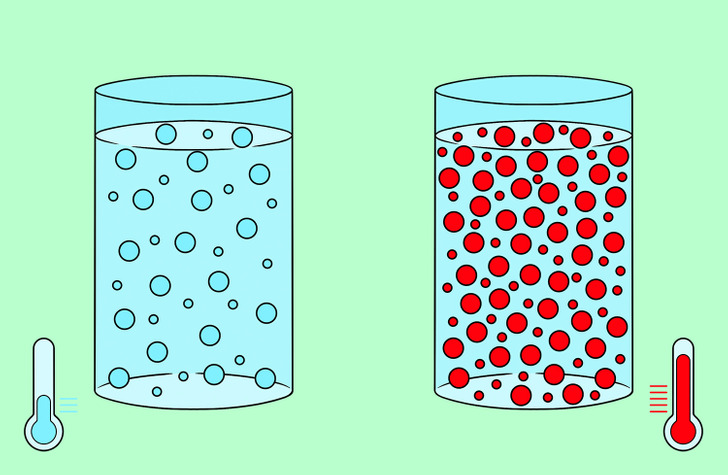
- Substance type. Particles of gases are more mobile, and diffusion in them occurs faster than in liquids and solids.
- Temperature. The hotter the gas or liquid is, the more energy the particles have and the faster diffusion occurs.
- Substance concentration. The more particles there are, the faster the diffusion rate is.
- Distance. The shorter the distance the particles propagate, the faster the diffusion rate will be.
- Particle size. The diffusion of a smaller particle will be more rapid than that of a larger-sized particle. This is related to the mass of the molecule and its surface area. A heavy molecule with a large surface area will mix with other particles slowly, while smaller, lighter particles will spread faster.
What role diffusion plays in our life
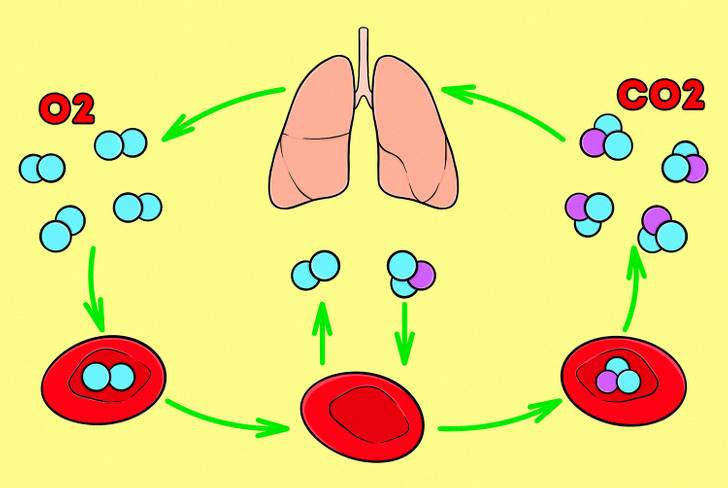
Many natural processes in our bodies are based on the diffusion of molecules. For example, breathing involves the diffusion of gases (oxygen and carbon dioxide) into and out of the blood. When air enters our body, red blood cells bind oxygen and carry it to tissues, where gases and nutrients are exchanged. In the process, carbon dioxide and waste products diffuse from tissue cells into the blood, and oxygen and nutrients diffuse from the blood into bodily tissues.
Even the production of oxygen we breathe depends on diffusion. Photosynthesis uses the energy from sunlight, water, and carbon dioxide to produce glucose, oxygen, and water. Carbon dioxide diffuses from the air through tiny pores in the leaves of plants called stomata. The oxygen that is generated this way diffuses from the plant through the stomata into the atmosphere.