Why Copper Turns Green

Copper is one of the few metals with a natural color that isn’t a gray-white hue. Its natural color is peach or pinkish-white, with a bright metallic shine. However, over time, it changes its color beyond recognition and becomes green.
5-Minute Crafts is going to explain to you why this metal loses its initial shade and turns green.
Why does copper turn green?

Copper acquires a greenish tint during the oxidation process that occurs when it comes into contact with air. In conditions where there is high humidity, oxidation happens faster, which results in the formation of a thin bluish-green outer layer called patina on the metal’s surface.
The color of the patina is due to the minerals that it consists of and which are formed on the surface of the metal during oxidation. One of them, brochantite, is green, the other one, malachite, is emerald blue, and the third one, azurite, is blue.
Patina can be compared with rust on iron which also forms during oxidation. However, unlike rust, patina doesn’t destroy the metal but conversely contributes to its protection.
Why patina means good?
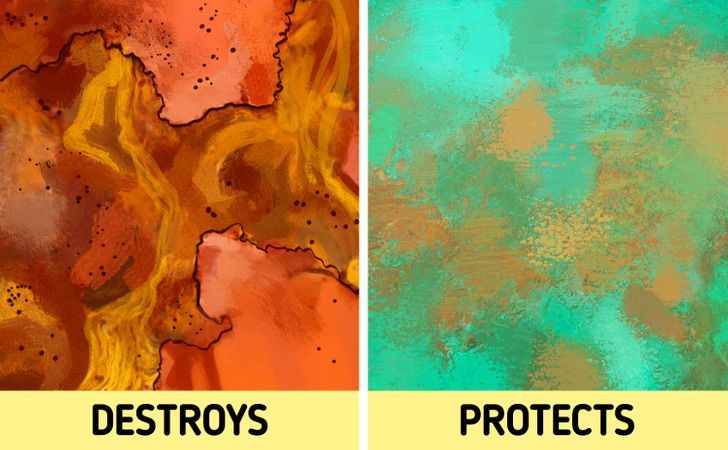
When iron is rusted, the top layer of the metal oxidizes and flakes off, thereby opening up the possibility of further oxidation. Over time, rust can penetrate into the metal and corrode entire structures.
Copper oxide is a strong substance that adheres very tightly to the base metal and prevents its further destruction. The thicker the patina layer becomes, the more it resists the corrosion of the metal underneath.
Perhaps, the most famous example of copper oxidation is the Statue of Liberty that was built in 1886. Despite being more than 100 years old, the layer of patina on the statue still doesn’t exceed 0.005 inches.

Which other metals turn green?
Metal alloys that contain copper are also subject to oxidation and patina. Over time, brass, which consists of copper and zinc, and bronze, which is made of copper and tin, acquire a greenish tint too.