What’s Smaller: an Atom or a Molecule?
Particles, in physics, can be either elementary or composite. Elementary particles don’t have an inner structure, which means there are no other particles inside them. Composite particles, on the contrary, have a complex inner structure and consist of particles of smaller sizes. Atoms and molecules refer to composite particles.
5-Minute Crafts is telling you what atoms and molecules are all about and which of these particles is smaller.
What an atom is

The word atom comes from the old Greek word, ἄτομος, which means “indivisible.” It’s the smallest unit that forms a chemical element.
Each atom consists of a core and elementary particles — one or several electrons surrounding the core. The core, in its turn, consists of one or several protons and several neutrons. More than 99% of the atom’s mass is in its core.
Atoms can attach to each other through chemical bonds and form molecules.
What a molecule is
The word, “molecule,” comes from the new Latin word, “molecula,” which is a derivative of another Latin word, “moles,” which means “mass.” In a scientific sense, the name reflects the understanding of a molecule as the smallest part into which a substance can be divided without violating its chemical properties.
In its structure, the molecule represents a group of 2 or more atoms held together by chemical bonds. Most of the organic and inorganic substances on the planet are molecules, such as water, sugar, vitamins, or amino acids that make up DNA.
What is smaller — an atom or a molecule?
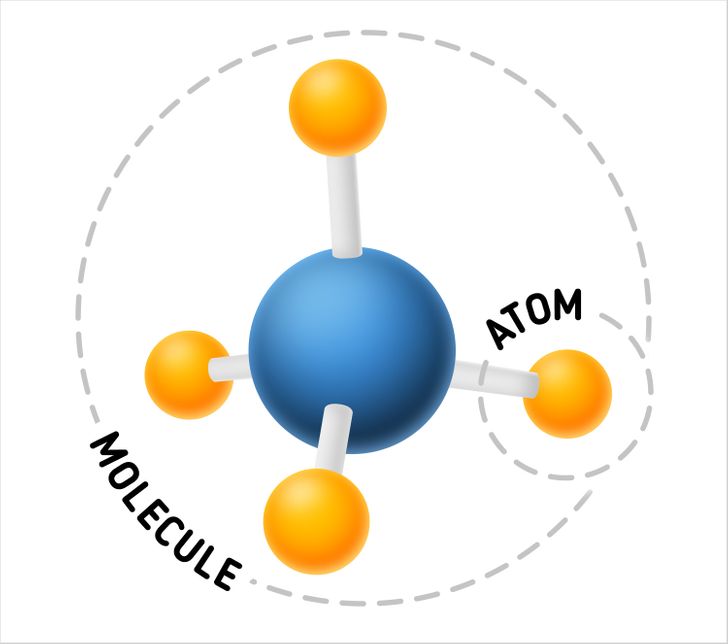
It’s an atom because it’s a compound part of a molecule and makes up a molecule’s structure together with other atoms.
Despite its smaller size, an atom, just like a molecule, refers to composite particles. Its structure, in its turn, is made up of elementary particles of a smaller size — protons, electrons, and neutrons.